The Higgins Laboratory

The Higgins laboratory is located on the third floor of Johnson Hall on the OSU campus.
The laboratory contains a laminar flow hood, carbon dioxide incubator, cell culture microscope and all other equipment necessary for cell culture.

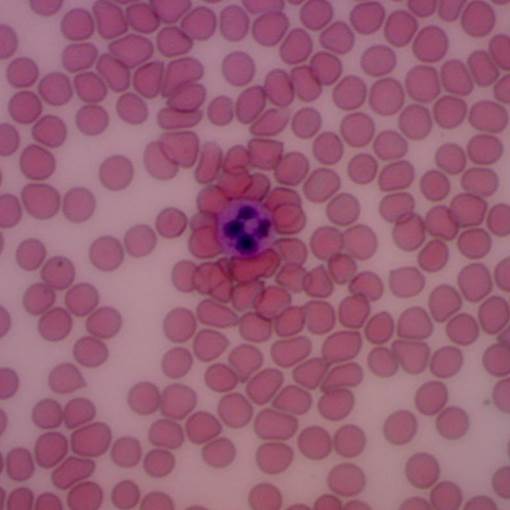
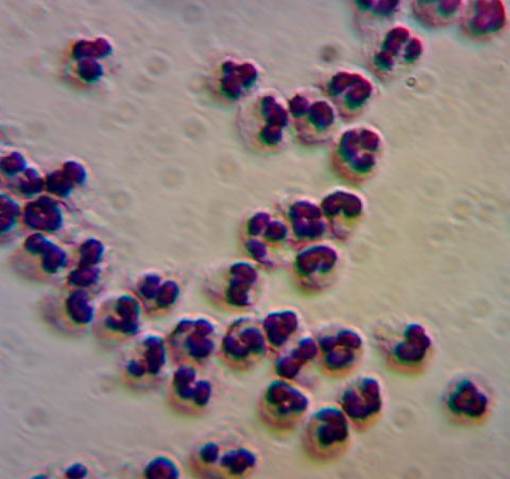
The laboratory is equipped for BSL-2 research and we have several IRB approved protocols for working with human blood. We have the equipment necessary for measuring hematocrit, hemolysis and cell counts within the lab, and we have access to clinical tests (complete blood counts, blood chemistry) through OSU Student Health Services. We have experience with isolation of cell subpopulations from blood, including T-cells and neutrophils.
The laboratory contains an upright microscope with long working distance optics and a high speed video camera for capturing the rapid movement of particles (e.g., red blood cells) within microfluidic devices. For fabrication of microfluidic devices we use the facilities at the Advanced Technology and Manufacturing Institute.

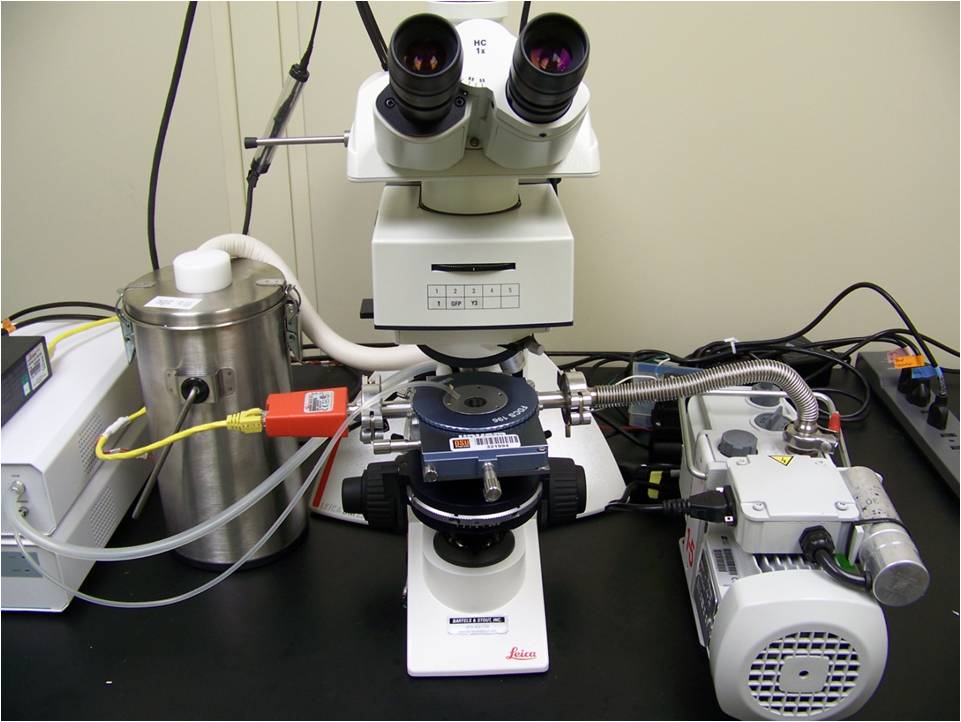
The cryomicroscopy system consists of a temperature-controlled microscope stage (pictured below), a customized upright microscope and a high-speed digital camera. It is capable of precise temperature control between -190 C and 120 C, cooling and heating rates of up to 130 C/min, and image acquisition rates of up to 8400 Hz (at a resolution of 256x256 pixels). We use the cryomicroscopy system to visualize the dynamics of the intracellular ice formation process. Because intracellular ice formation is a major mechanism of cell damage during cryopreservation, these observations are useful for designing cryopreservation procedures.
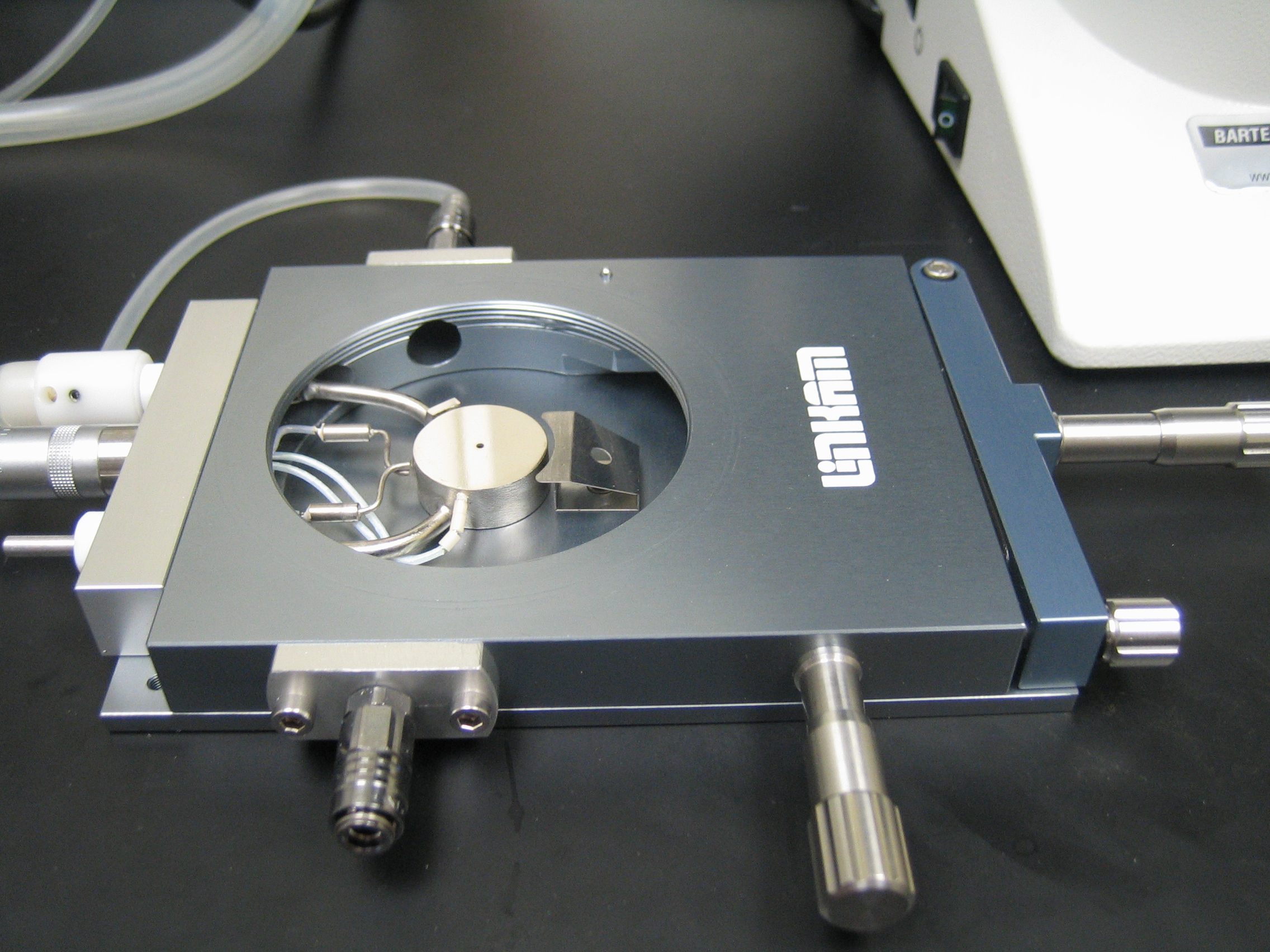
The freeze-drying microscopy setup allows microscopic observation of a thin sample throughout the freeze-drying process. The experimental setup is the same as the cryomicroscopy system, with the addition of a vacuum pump, bleed valve and pressure gauge for control of the vacuum pressure. It can be used to visualize the ice crystallization process, the drying rate and the stability of the freeze-dried matrix. In particular, it can be used to measure the cake collapse temperature, which is useful for optimizing primary drying procedures.
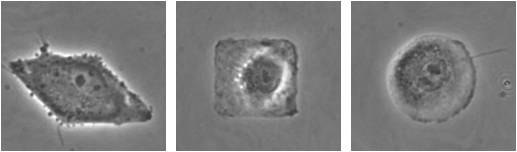
We have expertise and experimental capabilities in surface micropatterning to control the geometry of attached cells. The pictures at the right show single endothelial cells micropatterned in the shape of a diamond, square and circle.
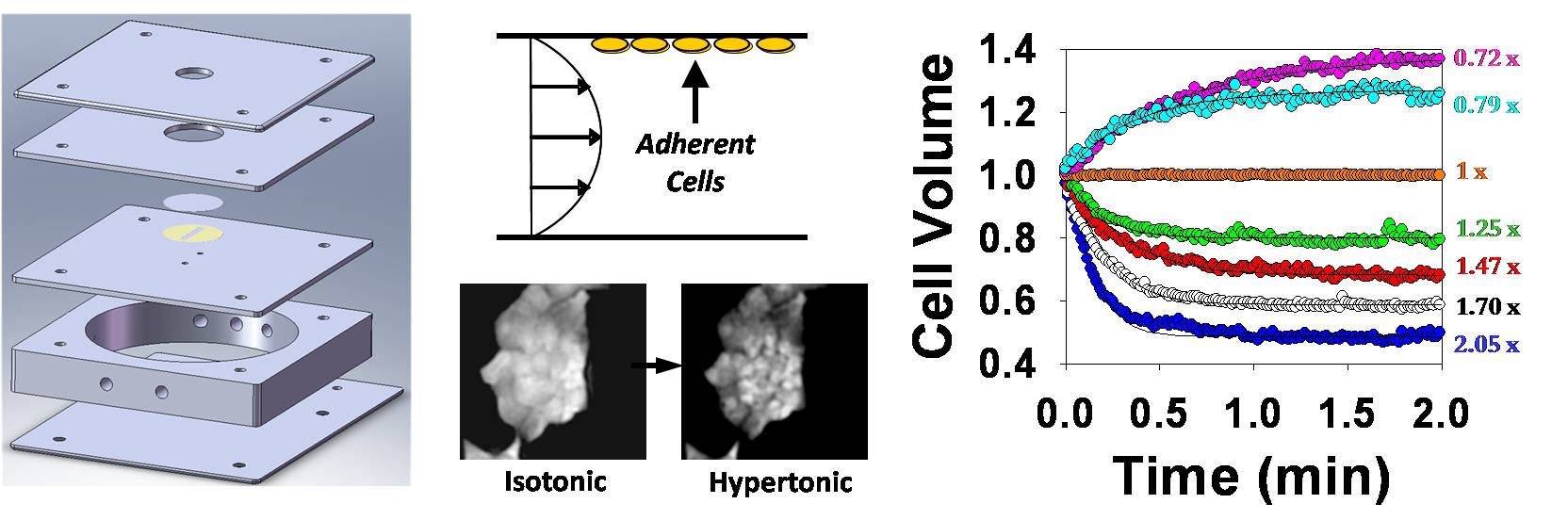
Cell membrane permeability properties are important for designing cell preservation procedures. The laboratory has a custom experimental setup for measuring membrane permeability in attached cells and 2D tissue using a fluorescence quenching technique. The technique involves measurement of cell-volume-dependent changes in fluorescence intensity by time-lapse fluorescence microscopy after exposure to anisotonic solutions in a flow chamber. The flow chamber (shown below) is capable of rapid solution exchange and has a built-in heat exchanger for precise control of temperature during permeability measurement.
Adam Higgins
School of Chemical, Biological and Environmental Engineering
Oregon State University
Corvallis, OR 97331
adam.higgins@oregonstate.edu
Phone: (541)-737-6245
Fax: (541)-737-4600